Abstract
IntroductionFor patients in need of an allograft and lacking an HLA-identical sibling, hematopoietic stem cell transplantation (HSCT) from an unrelated donor (UD) is the standard of care. In the recent few years, αβ T-cell and B-cell depleted HLA-haploidentical HSCT (αβhaplo-HSCT) is becoming a widely employed alternative to UD-HSCT in patients with acute leukemia. We, thus, decided to conduct a retrospective analysis on the outcome of patients given either αβhaplo-HSCT or UD HSCT in the same time period, namely October 2010-December 2015, in one of the 13 centers affiliated with the Italian-HSCT pediatric network.
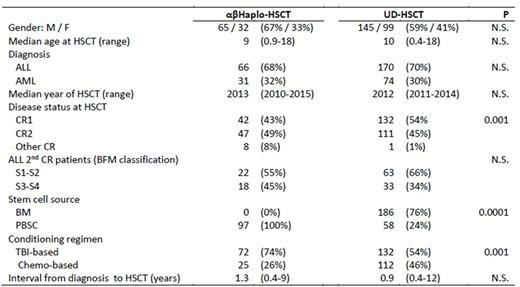
Methods: Included in the study were 97 αβhaplo-HSCT recipients and 244 patients receiving UD-HSCT in the same period. Six centers performed both αβhaplo-HSCT and UD-HSCT, while only this latter procedure was performed in the remaining 7 centers. All children were transplanted in morphological complete remission (CR) after a fully myeloablative conditioning regimen. The UD was selected using high-resolution typing for the HLA-loci A, B, C, DRB1. The UD was 8/8 HLA-matched with the recipient in 52% of cases, while the remaining 48% of patients were transplanted from a donor with either 1 or 2 HLA-disparities. Details on patient characteristics of the 2 groups are shown in the Table; recipients of αβhaplo-HSCT were transplanted in more advanced phase and received more frequently a TBI-based regimen than UD-HSCT patients. Patients given αβhaplo-HSCT did not receive any post-transplantation pharmacological prophylaxis of graft-versus-host disease (GvHD), while the combination of anti-T lymphocyte globulin (ATLG), cyclosporine-A and short-term methotrexate was employed for preventing GvHD occurrence in all UD-HSCT recipients. ATLG was also infused before transplantation in all children treated with αβhaplo-HSCT to prevent both graft rejection and GvHD.
Results: Two (2%) and 4 (2%) patients experienced graft failure in the αβhaplo-HSCT and UD-HSCT groups, respectively. Median time to neutrophil and platelet recovery was shorter in children given αβhaplo-HSCT (13 and 11 days vs. 19 and 23 days, respectively, P < 0.001 in both cases). The cumulative incidence (CI) of grade II-IV and grade III-IV acute GvHD in patients given αβhaplo-HSCT was 16% and 0%, as compared to 39% and 12% in UD-HSCT recipients (P < 0.001 and < 0.0005, respectively). Children treated with αβhaplo-HSCT benefited also from a lower incidence of both overall and extensive chronic GvHD (6% and 1%, respectively, vs 20% and 7% in UD-HSCT recipients, P < 0.01 and < 0.05, respectively), especially when compared with 7/8 and 6/8 UD-HSCT recipients. Forty-eight patients died for transplant-related complications: 9 (9%) and 40 (16%) in the αβhaplo-HSCT and UD-HSCT group, respectively, the CI of non-relapse- mortality (NRM) being 9% and 17% (P = N.S.). While the probability of NRM is superimposable in αβhaplo-HSCT and 8/8 UD-HSCT, 7/8 and 6/8 UD-HSCT had a higher risk of mortality (25% and 37%, respectively). Seventy-three children relapsed at a median time of 190 days (range 40-1603) after the allograft; no statistically significant difference for the CI of disease recurrence was observed between the 2 groups (25% vs 20%, respectively). With a median follow-up of 3.3 years (range 0.5-5 years for surviving patients) the 3-year probability of overall survival for patients given either αβhaplo-HSCT or UD-HSCT is 68% and 64%, respectively (P = N.S.), while that of event-free survival is 63% and 62%, respectively (P = N.S.). The 3-year chronic GvHD-free/relapse-free survival (GRFS) in the 2 groups is 59% and 48%, respectively (P = 0.03). GRFS of αβhaplo-HSCT or 8/8 UD-HSCT recipients was superimposable.
Conclusions: Patients givenαβhaplo-HSCT had a faster ANC/PLT recovery and a lower probability of acute GvHD in comparison to children transplanted from an UD donor. Moreover, they also had a lower risk of NRM and better probability of GRFS in comparison to patients transplanted from an UD with 1 or 2 HLA-disparities. αβhaplo-HSCT is associated with a CI of disease recurrence comparable to that of children transplanted from an UD. Altogether, these data indicate that αβhaplo-HSCT is a competitive alternative to UD-HSCT, especially in the absence of a fully-matched donor.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.